FRU CARESCAPE V100 V1.5 MAIN BD MS2011
| 2037103-087 | |
| Patient Monitoring | |
| GE HealthCare | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Very efficient
- Label has anti-static properties
- Good performance
Product Overview
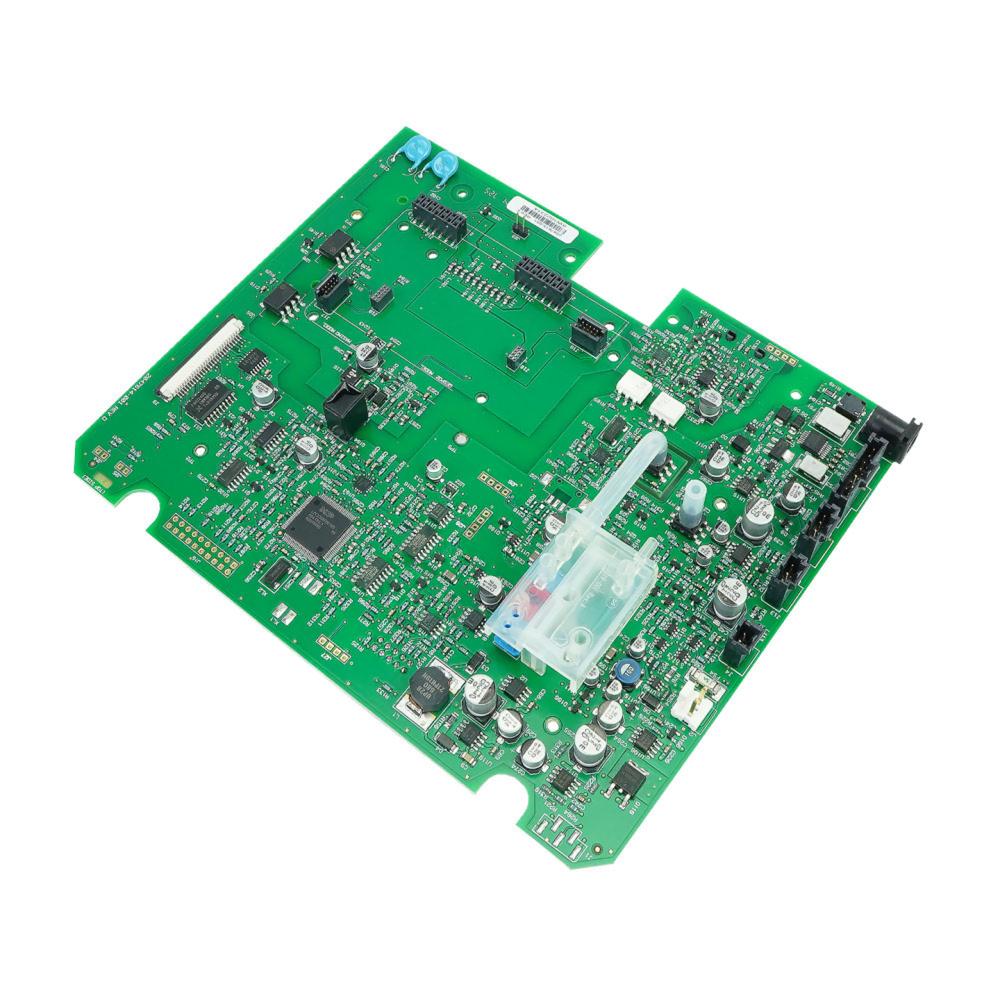
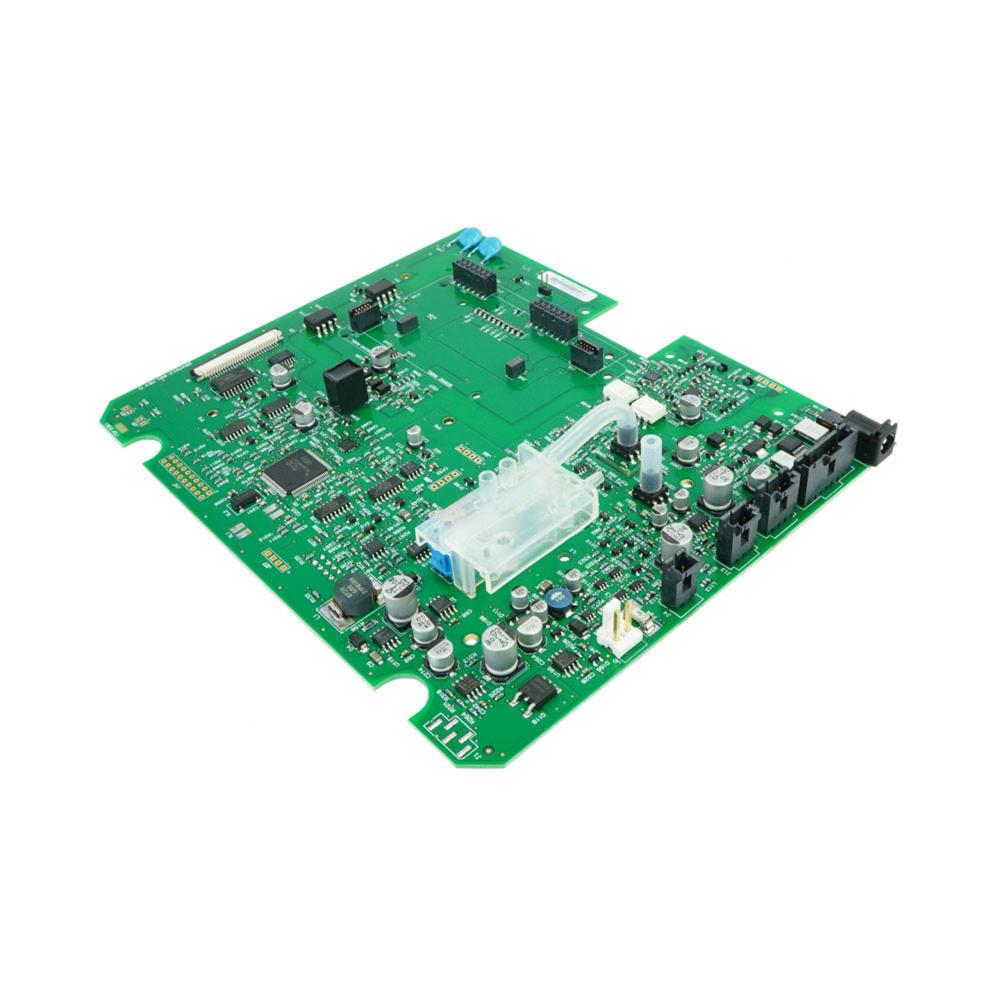
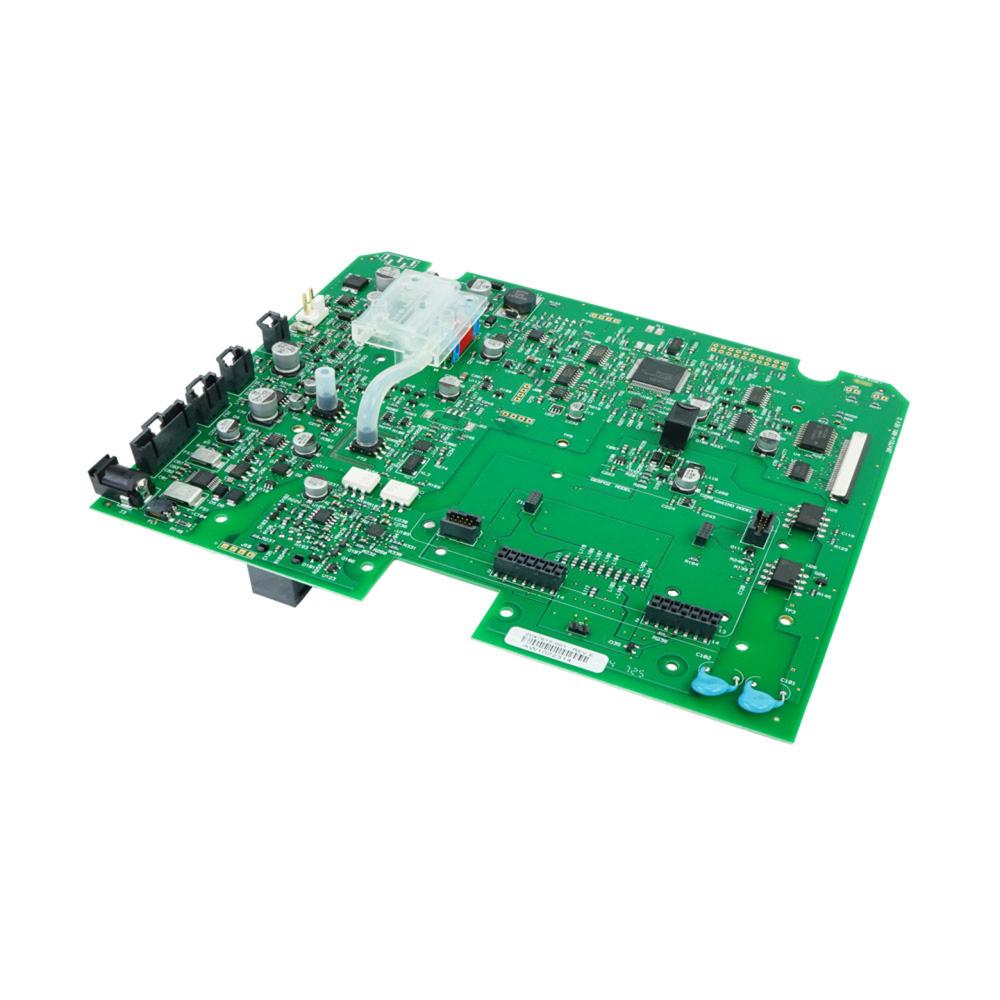
The Main Board or Motherboard is considered the most important hardware in the computer system. It is a printed circuit board that enables all of the hardware components to communicate with each other. It consists of the RAM, memory, processor, bus, slots and connectors. Main Board v1.5 MS2011 kit is used in the display devices and is compatible to V100 with SH6 serial numbers. It contains the v2 main board assembly, carton mailer foam and label. The v2 main board assembly consists of the main processor, power processor, NIBP measurement, battery circuit, main power supply, printer interface, audio alarms, host communications, display connector and isolated power supply. These circuits are fitted in the board to perform their functions, it includes IC's, resistors, capacitors and batteries. The main board is packed in single wall B flute corrugated fiberboard which has an antistatic foam pad and a convoluted pad. The foam has dimensional tolerance of ± 0.4. All burs and sharp edges have been removed. The label has an anti-static feature and is adhesive backed.