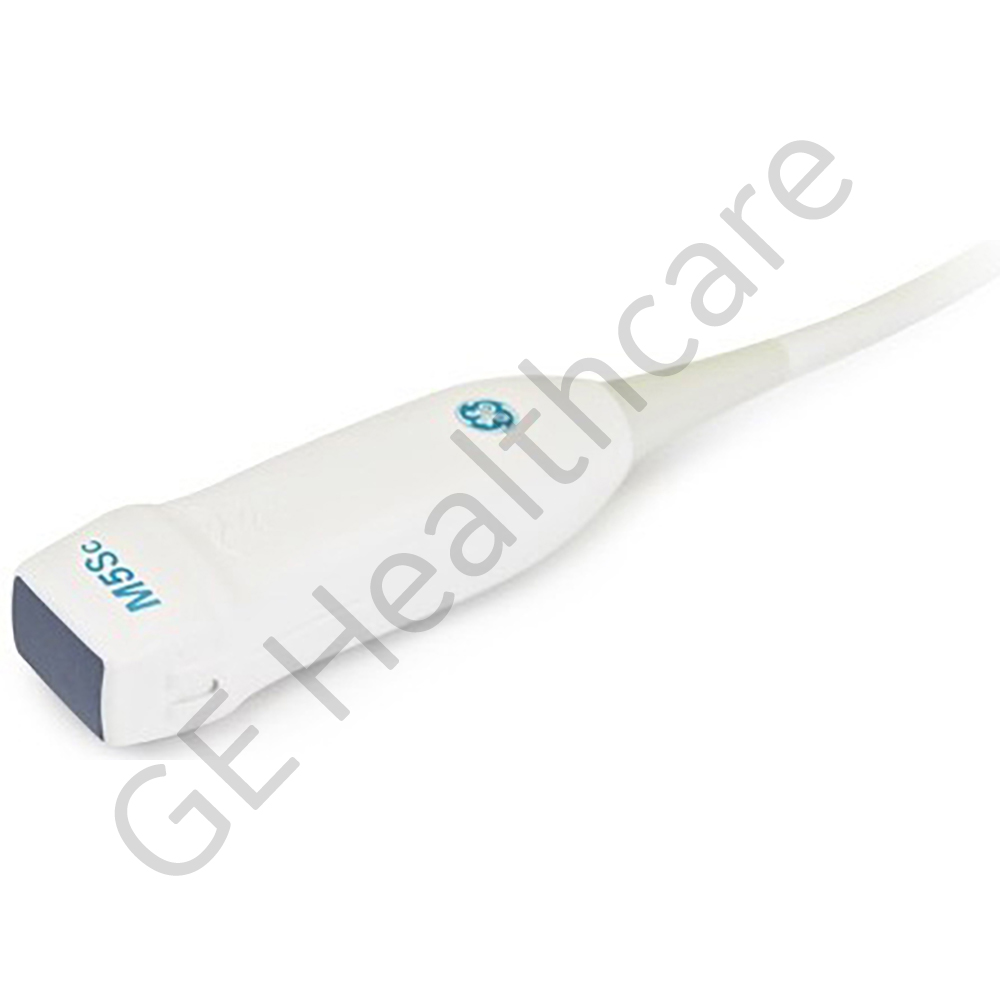

M5Sc-D, PROBE TYPE
| 5446030 | |
| 5446812 | |
| Ultrasound | |
| VIVID E90 | |
| GE HealthCare | Exchange - Return Defective |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- High quality sector probe
- Durable
- Easy connection
- Aesthetic design
Product Overview
The M5SC-D Probe is a specially designed probe used for cardiac, pediatric abdomen, fetal heart and transcranial. The GE M5Sc-D Phased Array Transducer is compatible with the GE Vivid E9 ultrasound System The probe generates sector or pie-shaped images on the screen. The part is made of high quality material which provides reliable operation, efficient functioning and longer life span, which in turn makes the product well suited for many modern medical applications. The material also maintains the excellent toughness and has an attractive appearance. The transducers or probes are equipped with piezoelectric material which provides increased bandwidth, enhanced signal to noise and enhanced axial resolution and penetration. Uniform resolution throughout the field of view is also achieved using multiple rows of crystals in matrix array technology. The part also offers easy handling to mount or attach. The part is diligently designed for superior performance and reliability. The GE product is an innovation and technology which fits well into versatile customer needs.
의료기기법상 허가/신고 번호
1. 수인 07-522호(Voluson E6/E8/E10)의 구성품
2. 수인 09-209호(Vivid E9)의 구성품
3. 수인 15-932호(Vivid S70/S60)의 구성품
4. 수인 15-1089호(Vivid E80/90/95)의 구성품
5. 수인 16-4615호(Vivid S60N/S70N)의 구성품
6. 수인18-4342호(LOGIQ E10)의 구성품
Additional Features
- 120° field of view
- Uniform image resolution
- Comfortable handling
- Flexible and long lasting
- Lightweight
- Excellent image quality
- Used for adult and children patients
- Tested to UL IEC and ISO safety standards
- Environmental contaminant free - no lead material used
- RoHS compliant
- GSPO
Equivalent Item(s):
Below is more information on the equivalent item(s). Items without a hyperlink are listed for reference only and are not available for purchase online.
| Equivalent Item(s) | Item Details |
|---|---|
| 5446812 | M5Sc-D PROBE |